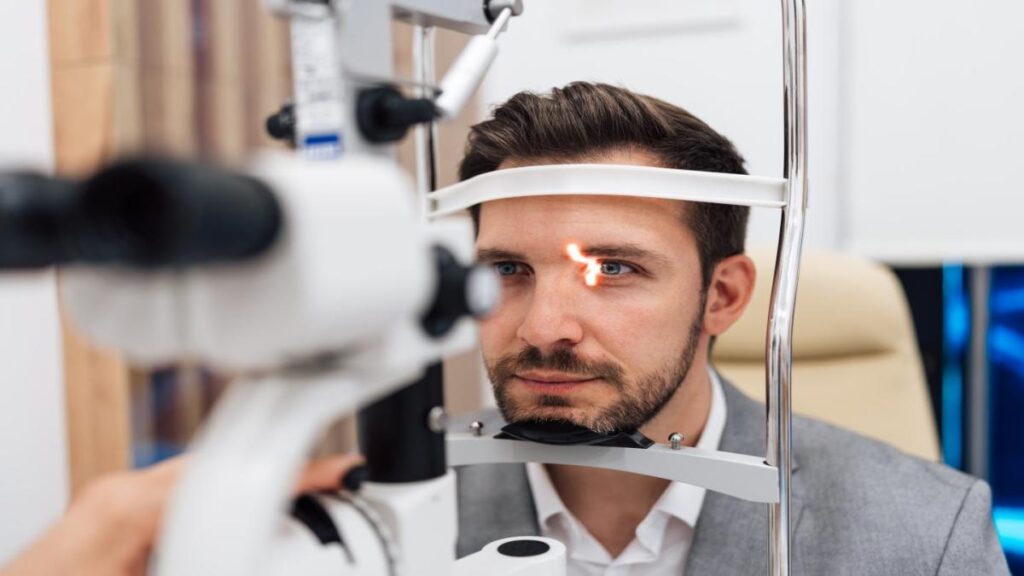
The U.S. Food and Drug Administration (FDA) has officially authorized LumiThera’s Valeda Light Delivery System for the treatment of dry age-related macular degeneration (AMD), marking a significant advancement in therapeutic options for patients experiencing vision loss due to this condition. The Valeda system is reported to enhance best corrected visual acuity (BCVA) by over five...
Previous ArticleDonald Trump’s Belief in Influencing Fed Rates: Insights from History and Legal Perspectives
Next Article Key Investment Strategies for a Trump or Harris Win
Related Posts
Subscribe to Updates
Get the latest news and updates directly to your inbox.
© 2025 Arcalis News. All Rights Reserved.